Defective St. Jude ICD / CRT-D Devices – Early Battery Depletion - Long Island Accident Attorneys | RGLZ Personal Injury Law
Health Management and Leadership Portal | Implantable cardiac stimulator / cardioverter-defibrillator / automatic Ellipse™ ICD St. Jude Medical | HealthManagement.org

MRI Safety for Patients Implanted With the MRI Ready ICD System: MRI Ready Study Results - ScienceDirect

CD2357-40Q, St. Jude Medical, Implantable Cardioverter Defibrillator, Fortify Assura DR 40, Dual-chamber ICD with RF telemetry, Connector DF4-LLHH/IS-1
Manufacturer : St Jude Medical Product name : Ellipse VR/DR Implantable Cardioverter Defibrillators (ICD) Product code : Ellipse
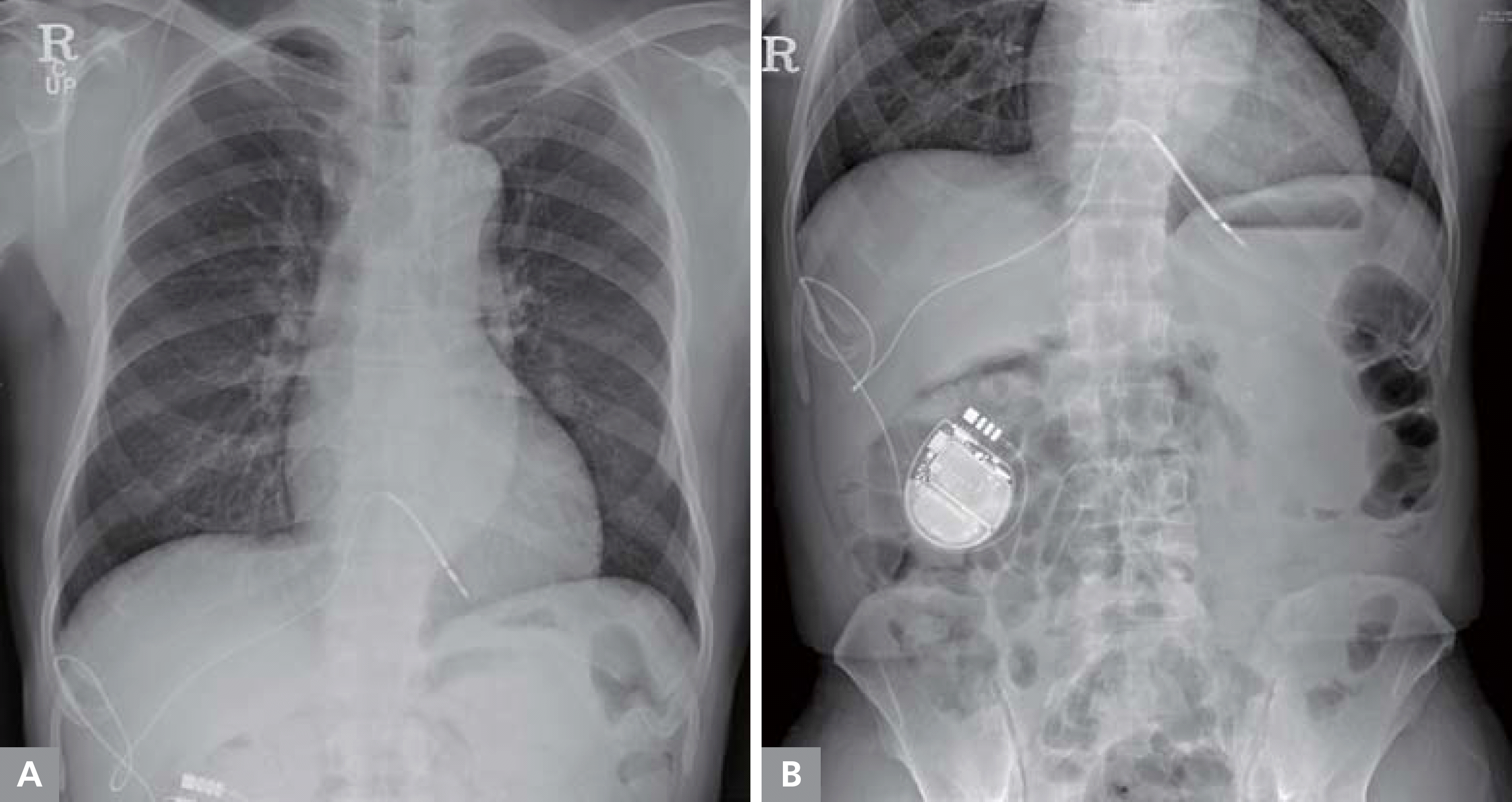
Successful detection of a high-energy electrical short circuit and a “rescue” shock using a novel automatic shocking-vector

CD1411-36C, St. Jude Medical, Implantable Cardioverter Defibrillator, Ellipse VR 36, Single-chamber ICD with RF
Registration Number MDR-10617A Product Name ELLIPSE™ VR SINGLE CHAMBER IMPLANTABLE CARDIOVERTER DEFIBRILLATOR (ICD) Manufactur

Inappropriate defibrillator shock due to EMI despite noise reversion and SecureSense - What is the mechanism? - ScienceDirect